Making Intravascular Diagnosis and Treatment More Accurate and Faster: Intelligent Guidewire Design
Guidewire is one of the most basic instruments in vascular interventional procedures, mainly used as an access type of consumable to reach the target location and establish access to facilitate the delivery of other diagnostic and therapeutic catheters (e.g. microcatheters, balloon dilatation catheters, stent delivery catheters).
With more and more sensors/transducers being applied to interventional devices, various kinds of intelligent catheters with diagnostic and therapeutic functions have been widely used, such as IVUS catheters and FFR catheters for diagnostic purposes, and radiofrequency ablation catheters, cryoablation catheters and PFA ablation catheters for therapeutic purposes.
Unlike a catheter, a guidewire is an essential access-type consumable with a convenience that a catheter cannot match.
If a diagnostic or therapeutic function can be performed on a guidewire, the:
1. Instrument delivery is more convenient and can reach a wider area;
2. At least one diagnostic or therapeutic catheter can be eliminated, with significant time and cost advantages;
3. If a diagnostic guidewire is used in conjunction with a therapeutic catheter, diagnosis and treatment can be synchronised, and treatment is more precise and rapid;
We are seeing more and more smart guidewires with diagnostic or therapeutic functions coming to market in addition to FFR pressure guidewires, such as SmartWire, a dual-sensor pressure guidewire for measuring aortic regurgitation developed by Xenter:
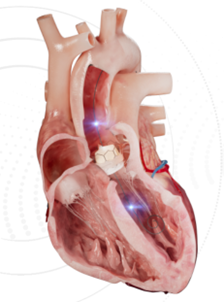
For example, SoundBite has developed a shockwave guidewire for the treatment of CTO lesions:
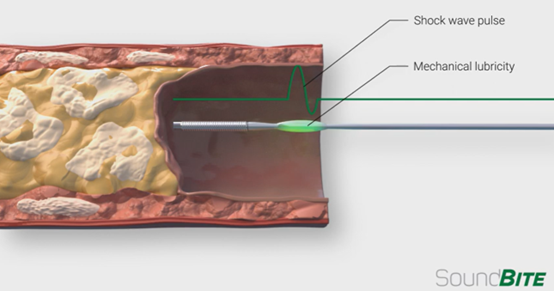
However, the much smaller size of the guidewire poses a significant challenge if additional sensors/transducers are to be installed.
Requirements for guidewire performance
Flexibility
Pushability
Steerability
Torqueability
In addition to the above properties that need to be focused on for guidewires, which need to be considered from a comprehensive perspective that includes proximal shaft, core, coil & cover, coating, tip, what is even more critical for smart guidewires is the design of the sensors and power wires, and the resultant impact on the established guidewire structure.
For example, smart guidewires mainly install power wires by narrowing or hollowing out the core wire, but these designs may make the guidewire's pushability, torqueability, and resistance to bending compromised.
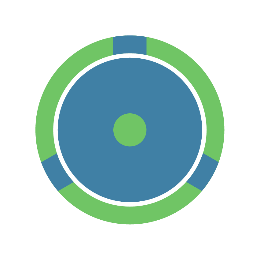

In addition to the modification of the core, it is also possible to design the wire outside the core, e.g. spiral wound and fixed on the surface of the core, e.g. embedded in the outer material.
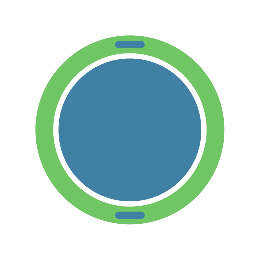
Although more specific requirements are placed on the material of the wire as well as the processing, the core can be better protected, as well as the mechanical properties of the guidewire.
Requirements for sensors/transducers
Biocompatibility & Sterilisation Compatibility: The material must meet the biocompatibility and stand up to disinfection and sterilisation methods.
Compliance: Sensors/transducers need to comply with medical device regulations and industry standards, ISO standards.
Protection and durability: Must be able to withstand external interference encountered during surgery, such as the use of coatings or housings.
Size and shape: small, thin and flexible to ensure smooth access to the target lesion with minimal trauma.
Sensitivity and range: the delay should be short to allow real-time measurements; the sensitivity should be high to detect small changes, and the range should be large to allow monitoring under various physiological conditions.
Transmission reliability: stable signal/energy transmission with external devices, wired/wireless solutions, wireless solutions need to consider power consumption and battery life.
Salt Solutions
The Salt Medical team has extensive experience in the design, development and manufacture of guidewires for a wide range of interventional diagnostic and therapeutic applications. We have the ability to integrate a wide range of diagnostic and therapeutic components, especially precision, flexible sensors, as well as processes such as guidewire grinding, welding, and spring winding, to fulfil a wide range of clinical needs.
Photo source: Xenter's website, SoundBite's website
About Us
Salt Medical is an international medical device CDMO company with an established global R&D and manufacturing network. We consist of R&D centers in Ireland and the U.S., and large-scale manufacturing centers in Asia Pacific. Focusing on catheters, guidewires, medical endoscopes, surgical robots and other product areas, Salt has accumulated a wealth of experience in the design, development and production of innovative products in cardio, neuro and endovascular applications as well as digestive, respiratory and other clinical areas. We utilise a fully synergistic network to provide the most cost-effective and responsive services to global medical device manufacturers.
